军团菌属(Legionella)是一类需氧革兰阴性杆菌,广泛分布于天然淡水、人工供水系统(如沐浴器、喷水池、冷却水塔)、空调制冷装置、车辆的雨刷水箱及单细胞原生动物(如阿米巴变形虫)细胞内[1]。目前,该属已发现60多个菌种,约一半以上菌种可对人致病[2],其中嗜肺军团菌(Legionella pneumophila)的致病力最强。免疫力低下的人群因吸入含嗜肺军团菌的气溶胶而引起人类军团病(Legionella disease),又称退伍军人症。军团病是一种可致命的急性发热性呼吸道传染病,由于首次暴发于1976年美国费城召开的一次退伍军人大会期间而得名,此后陆续在全球出现多次小范围的暴发流行[3-5]。根据患者的临床表现,军团病可分为庞蒂亚克热(Pontiac fever)和军团菌肺炎(Legionella pneumonia)两种类型。庞蒂亚克热是一种非肺炎型军团病,主要表现为急性流感综合征,无明显肺部病变,通常3~5 d即可自愈。军团菌肺炎是一种以肺部病变(如化脓性支气管炎、大叶性肺炎伴小脓肿形成)为主的急性全身性疾病,可由肺部迁徙入血引起播散,导致皮肤、肌肉、消化、神经等多系统损伤[6]。军团菌肺炎发病迅速、病死率高,已引起医学界的广泛关注。
1 嗜肺军团菌的胞内生存机制嗜肺军团菌可在营养较少的污水中存活数月甚至一年,且易与水中其他微生物相互作用形成混合菌生物被膜(biofilm)[7-9]。此时的嗜肺军团菌生存能力较强,但对宿主细胞的感染能力则相对较弱,处于“活而不可养”(viable but non-culturable, VBNC)状态[10-11]。自由生活阿米巴(FLA)能以细菌为食,被称作微生物界的“特洛伊木马”;而抗阿米巴菌能在FLA胞内存活与增殖,引起人类致病菌的扩散和传播[12]。嗜肺军团菌是一种典型的兼性胞内菌和抗阿米巴菌,能通过FLA (如棘阿米巴、福氏耐格里阿米巴)的吞噬而在其细胞中存活和增殖[13]。军团菌除了可以在阿米巴等单细胞原始动物胞内寄生和增殖外,还可以侵染哺乳动物的肺泡上皮细胞(epithelial cell)和单核巨噬细胞(macrophage cell),并在其中增殖,最终导致宿主细胞死亡。
嗜肺军团菌被阿米巴捕食后,迅速被封入由线粒体和细胞囊泡包绕的吞噬囊泡中,这个含有嗜肺军团菌囊泡体的结构被称作Legionella-containing vacuole(LCV)。嗜肺军团菌分泌的效应蛋白(effector)可调控宿主胞内运输途径和内外环境。吞噬起始阶段,LCV表面因带有宿主线粒体核糖体成分始终不会被酸化,同时募集内质网囊泡输送的蛋白并与之连接[14]。吞噬最后阶段,LCV膜融入粗面内质网中被多层粗内质网膜所包围,形成一种内体阻滞吞噬体(endosomal maturation-blocked, EMB) [15-16]。EMB的形成进一步抑制了LCV与溶酶体的融合,而避免被降解消化。
侵染早期,嗜肺军团菌在效应蛋白的作用下,干扰宿主的细胞周期,抑制宿主细胞的凋亡,创造出理想的胞内增殖环境,帮助军团菌从宿主细胞中逃逸与增殖[17-18]。侵染后期,随着嗜肺军团菌在EMB中不断地消耗宿主营养物质并大量增殖,最终涨破吞噬泡。嗜肺军团菌分泌的某些效应蛋白可诱导宿主细胞凋亡,使宿主细胞裂解死亡,从而从宿主胞内成功释放[19]。
2 嗜肺军团菌的致病物质嗜肺军团菌产生的酶类(肽基脯氨酰基异构酶、磷酸酯酶、DNA酶、蛋白水解酶等)、毒素(内毒素、细胞毒素等)和溶血素可造成宿主细胞损伤。肽基脯氨酰基异构酶可增强侵染巨噬细胞的能力[20];磷酸酯酶可抑制中性粒细胞超氧化物阴离子的产生,而后者可作为第二信使参与免疫应答等生理功能;细胞毒素可影响中性粒细胞的氧化代谢;脂多糖可促进嗜肺军团菌对宿主细胞的黏附,并具有促炎作用,引起肺部感染[21-22]。军团菌产生的这些致病物质还可对抗宿主的免疫杀伤,使其在吞噬囊泡中存活并增殖,最终导致宿主细胞死亡[23]。嗜肺军团菌内毒素除引起高热、低血压、弥散性血管内凝血、内毒素休克等革兰阴性内毒素相同生理病理反应外,还可通过激活补体系统,促进嗜肺军团菌进入单核巨噬细胞胞内[24]。此外,菌毛的黏附作用,微荚膜的抗吞噬作用也参与其发病过程。
嗜肺军团菌可以在宿主细胞内存活主要是依赖其独特的毒力分泌系统向宿主胞内注入分泌型效应蛋白。嗜肺军团菌具有I、II、IVA、IVB及V型分泌系统[25],其中IVB型分泌系统(type IVB secretion system, T4BSS)在侵染宿主的过程中发挥了至关重要的作用[26-27]。T4BSS分泌系统又被称作Dot/Icm分泌系统,主要由2个致病区域(共7个基因和18个Icm)组成。其中Dot代表细胞器转运缺陷基因(defective organelle trafficking gene),Icm代表细胞内繁殖基因(intracellular multiplication gene)。嗜肺军团菌Dot/Icm IVB型分泌系统是其最主要的毒力系统,可向宿主细胞内注入300种以上的效应蛋白,而这些蛋白在其侵染宿主过程中发挥了重要生物学功能[28]。
3 嗜肺军团菌效应蛋白的主要功能嗜肺军团菌的毒力因子除普通胞外菌具有的侵袭力和毒素外,还包括能影响其在宿主细胞内存活与增殖的所有菌蛋白[29]。嗜肺军团菌非效应蛋白的毒力蛋白包括:Fe2+转运蛋白FeoB [30]、鞭毛调控σ因子FliA[31]、磷脂酶A调控σ因子RpoS[32]、肽基脯氨酰基顺反异构酶Mip[33]、毒素RtxA [34]、促内吞蛋白EnhC[35]和热休克蛋白HSP60[36]等。Dot/Icm分泌系统效应蛋白作为主要的效应蛋白,由于其在致病过程中发挥重要作用,近年来相关研究逐渐增多。这些效应蛋白参与军团菌感染、复制、增殖和自噬的各个阶段,但是目前对大多数效应蛋白的功能研究相对较少,为了深入了解效应蛋白的功能,现将国内外研究较多的效应蛋白的生物学功能归纳如下。
3.1 形成保护性LCV,避免与溶酶体融合研究[37-38]表明:Dot/Icm系统缺陷的嗜肺军团菌突变株被宿主吞噬后,可与酸性LAMP1-阳性的溶酶体结合而被消灭;而Dot/Icm系统完整组装后,则可直接阻断与溶酶体的结合。嗜肺军团菌效应蛋白可通过俘获宿主胞内参与囊泡转运的重要蛋白,从而干扰宿主囊泡的转运;同时通过俘获宿主胞内的内质网-高尔基体囊泡,形成可保护自身的寄生性囊泡LCV使其不被溶酶体识别消化,帮助其在宿主胞内的存活与增殖[39-40]。这些效应蛋白包括SidM、SidD、LidA、LepB、RalF、AnkX/Lem3、VipA、VipD、SidC、SidE、SidJ、LseA、PieE、LegG1、RidL和YlfA/LegC7等[41]。
SidM (DrrA)具有鸟苷酸交换因子(GEF)和鸟苷酸解离抑制因子释放因子(GDF)双重活性,并可对Rab1蛋白特异性识别;而Rab蛋白作为一种分子开关,在囊泡的出芽、转运、黏附、锚定、融合的各阶段发挥重要作用[40, 42-43]。嗜肺军团菌SidM正是通过俘获介导宿主内质网-高尔基体转运的关键因子Rab1,使其形成成熟的LCV。首先SidM通过对Rab1的特异性识别,与AnkX和Lem3一起辅助解离Rab1-GDI复合物,并与LidA共同完成对Rab1的竞争性俘获和稳定,参与Rab1激活和AMP化修饰。在调控后期,SidD通过对Rab1的AMP化修饰,促进LepB识别并水解Rab1-GTP,使Rab1再次回到活性关闭状态,并从LCV上脱离且被胞内GDI回收,构成完整的膜循环[41, 44-46]。此外,VipA和VipD在嗜肺军团菌调控宿主囊泡转运过程中亦发挥了重要作用。VipA通过参与肌动蛋白的结合与聚合而调控囊泡转运,VipD通过阻止一些小G蛋白(如Rab5、Rab22)进入正常的膜循环,抑制宿主溶酶体成熟[47-48]。
3.2 激活抗凋亡NF-κB通路,抑制宿主细胞凋亡嗜肺军团菌感染早期需要在宿主细胞内大量繁殖从而侵染细胞,因此,宿主细胞的过早凋亡对军团菌的增殖与侵染极其不利。致病性嗜肺军团菌可通过向宿主胞内分泌效应蛋白调控宿主抗凋亡基因的表达,来抑制细胞凋亡或延长细胞的存活时间。例如,可以通过正向调节NF-κB通路(细胞免疫反应的主要调控因子)以延缓宿主细胞的凋亡。研究[49-50]表明:Dot/Icm底物效应蛋白LegK1、LnaB、SdhA、sidF和LubX等可能参与激活宿主细胞抗凋亡NF-κB通路。例如,丝氨酸/苏氨酸蛋白激酶(STPK) LegK1,能模拟宿主的IκB而激活宿主的NF-κB信号转导通路,在宿主细胞中显示出很强的NF-κB信号活性,从而能起到防止宿主细胞过早凋亡的作用。某些蛋白激活宿主NF-κB活性的机制尚不完全明确,可能是多个效应蛋白协同作用的结果。
3.3 促使宿主细胞死亡,从宿主细胞中逃逸嗜肺军团菌生命周期的最后一步是成功从宿主细胞中释放出来。LepA和LepB是两个具有卷曲螺旋结构域(coiled-coil domain)的效应蛋白,可能参与宿主裂解死亡过程。研究[51-52]发现:嗜肺军团菌LepA和LepB缺失株与野生株相比,在宿主细胞内的增殖能力无显著差异,而从原生动物细胞中释放的能力却显著下降。这些研究说明LepA和LepB很可能在裂解宿主细胞的过程中发挥了重要的作用,可帮助嗜肺军团菌从宿主细胞中成功逃逸。
3.4 带有真核蛋白序列基元效应蛋白的生物学意义嗜肺军团菌基因组特征分析发现其基因组相当大,可编码大量类真核生物蛋白或编码的蛋白具有真核生物蛋白的保守结构域。例如,上文提到的STPK LegK1可通过激活宿主的NF-κB信号转导通路而抑制宿主细胞过早凋亡;STPK LegK3可引起较弱的酵母菌生长抑制且可调节酵母菌属的囊泡转运[53]。通过观察酵母胞内LegK3与肌动蛋白细胞骨架和内质网到高尔基体的小泡之间的共同定位现象,可以发现他们之间存在相关作用[54],但具体作用机制尚不明确。嗜肺军团菌可表达多种真核效应蛋白,这些蛋白可介导蛋白质间的相互作用,是一类潜在的毒力因子[55]。
3.5 效应蛋白的其他生物学功能嗜肺军团菌某些效应蛋白还具有其他方面的生物学功能。例如:RomA含有甲基转移酶的SET结构域,可甲基化修饰宿主细胞核质,抑制宿主基因表达[56];LegU2具有U-box结构域,具有E3泛素化连接酶的活性,介导泛素化细胞周期蛋白Clk1,阻碍宿主细胞周期进程[57];黏附相关效应蛋白SdeA可促进细菌内化[58];鞭毛蛋白FliC (FlaA)可促进细胞炎性坏死(又称细胞焦亡)和细菌释放[59-60];SidK通过靶向结合宿主v-ATP酶,抑制ATP水解,阻止液泡酸化[61];RidL可与内吞体分选(endosomes sorting)重要复合物Retromer结合,抑制其介导的囊泡物质转运,以帮助嗜肺军团菌的生存与增殖[62];SdeA能以一种特殊方式催化泛素化(蛋白质翻译后修饰方式之一)过程[63]等。
嗜肺军团菌除能产生酶类、毒素、菌毛和微荚膜等致病物质外,还具有独特的Dot/Icm IVB型毒力分泌系统,能够向宿主细胞分泌超过300种效应蛋白,而这些效应蛋白在其致病过程中亦发挥着重要的生物学功能,被列为广义的毒力因子。
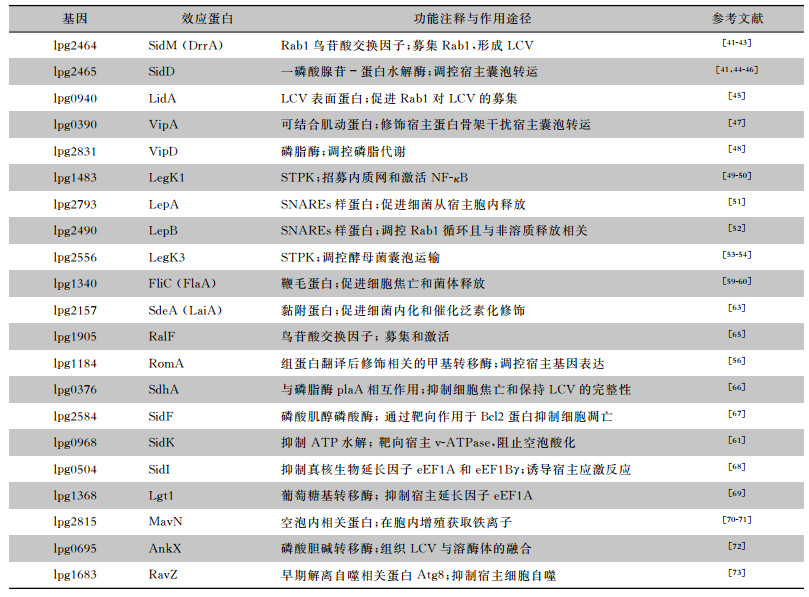
这些效应蛋白的生物学功能主要表现在以下三个方面:(1)侵染早期形成保护性LCV,有效避免与溶酶体融合而得以在胞内存活与增殖。(2)侵染过程通过激活宿主NF-κB通路抗宿主细胞凋亡;通过抑制宿主细胞的凋亡增加对其营养物质的利用,可以在宿主胞内更长久的存活与增殖。(3)侵染后期,通过裂解LCV或宿主细胞,促进宿主细胞凋亡或焦亡等方式帮助嗜肺军团菌从宿主细胞中顺利释放,实现进一步侵染。参考文献[64]现将报道较多的嗜肺军团菌效应蛋白归纳总结,见表 1,含文中未提及的SidI、Lgt1、MavN和RavZ等效应蛋白。
| 表 1 嗜肺军团菌效应蛋白在胞内增殖过程中对宿主细胞的作用途径 |
|
嗜肺军团菌是一种可引起军团病(包括高致死性军团菌肺炎和庞蒂亚克热)的病原菌。我国已将军团病列为需要重点防治的一种新发传染病。因此,针对嗜肺军团菌开展致病机制相关研究十分必要。
当前,嗜肺军团菌绝大多数效应蛋白的晶体结构和生物学功能尚未完全阐明,且效应蛋白在调控表达的信号通路、与宿主靶蛋白及宿主各调控信号网络的相互作用等方面的研究尚在起始阶段。全面深入地研究这些效应蛋白对于详细阐明嗜肺军团菌的致病机制具有重大意义,也为军团病的防治提供理论基础和新的药物靶标。此外,嗜肺军团菌作为一种研究胞内病原菌致病机制的理想模型,可为结核分枝杆菌、伤寒沙门菌等胞内菌与宿主先天性免疫系统间的复杂信号调控网络研究给予重要启示。
| [1] |
Mou Q, Leung PHM. Differential expression of virulence genes in Legionella pneumophila growing in Acanthamoeba and human monocytes[J]. Virulence, 2018, 9(1): 185-196. DOI:10.1080/21505594.2017.1373925 |
| [2] |
Wang H, Lu J, Li K, et al. The virulence of Legionella pneumophila is positively correlated with its ability to stimulate NF-κB activation[J]. Future Microbiol, 2018, 13: 1247-1259. DOI:10.2217/fmb-2018-0051 |
| [3] |
Mcdade JE, Shepard CC, Fraser DW, et al. Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory disease[J]. N Engl J Med, 1977, 297(22): 1197-1203. DOI:10.1056/NEJM197712012972202 |
| [4] |
Fraser DW, Tsai TR, Orenstein W, et al. Legionnaires'disease: description of an epidemic of pneumonia[J]. N Engl J Med, 1977, 297(22): 1189-1197. DOI:10.1056/NEJM197712012972201 |
| [5] |
Huang HH, Zhang YY, Xiu QY, et al. Community-acquired pneumonia in Shanghai, China: microbial etiology and implications for empirical therapy in a prospective study of 389 patients[J]. Eur J Clin Microbiol Infect Dis, 2006, 25(6): 369-374. DOI:10.1007/s10096-006-0146-7 |
| [6] |
Chitasombat MN, Ratchatanawin N, Visessiri Y. Disseminated extrapulmonary Legionella pneumophila infection presenting with panniculitis: case report and literature review[J]. BMC Infect Dis, 2018, 18(1): 467. DOI:10.1186/s12879-018-3378-0 |
| [7] |
Declerck P. Biofilms: the environmental playground of Legionella pneumophila[J]. Environ Microbiol, 2010, 12(3): 557-566. DOI:10.1111/j.1462-2920.2009.02025.x |
| [8] |
Boamah DK, Zhou G, Ensminger AW, et al. From many hosts, one accidental pathogen: the diverse protozoan hosts of Legionella[J]. Front Cell Infect Microbiol, 2017, 7: 477. DOI:10.3389/fcimb.2017.00477 |
| [9] |
Mampel J, Spirig T, Weber SS, et al. Planktonic replication is essential for biofilm formation by Legionella pneumophila in a complex medium under static and dynamic flow conditions[J]. Appl Environ Microbiol, 2006, 72(4): 2885-2895. DOI:10.1128/AEM.72.4.2885-2895.2006 |
| [10] |
Ducret A, Chabalier M, Dukan S. Characterization and resuscitation of 'non-culturable'cells of Legionella pneumophila[J]. BMC Microbiol, 2014, 14: 3. DOI:10.1186/1471-2180-14-3 |
| [11] |
陆勇军, 李向辉, 曾咏伦. 一个细菌的致命之旅——嗜肺军团菌分泌系统及效应蛋白的研究进展[J]. 遗传, 2011, 33(10): 1093-1101. |
| [12] |
Barker J, Brown MR. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment[J]. Microbiol, 1994, 140(Pt 6): 1253-1259. |
| [13] |
Fields BS, Benson RF, Besser RE. Legionella and Legionnaires' disease: 25 years of investigation[J]. Clin Microbiol Rev, 2002, 15(3): 506-526. DOI:10.1128/CMR.15.3.506-526.2002 |
| [14] |
Hoffmann C, Finsel I, Hilbi H. Purification of pathogen vacuo- les from Legionella-infected phagocytes[J]. J Vis Exp, 2012, 64: 3791-4118. |
| [15] |
Harb OS, Venkataraman C, Haack BJ, et al. Heterogeneity in the attachment and uptake mechanisms of the Legionnaires' disease bacterium, Legionella pneumophila, by protozoan hosts[J]. Appl Environ Microbiol, 1998, 64(1): 126-132. DOI:10.1128/AEM.64.1.126-132.1998 |
| [16] |
李勤学.自由生活阿米巴及其胞内菌的相互关系研究[D].上海: 复旦大学, 2005.
|
| [17] |
Laguna RK, Creasey EA, Li Z, et al. A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death[J]. Proc Natl Acad Sci USA, 2006, 103(49): 18745-18750. DOI:10.1073/pnas.0609012103 |
| [18] |
Banga S, Gao P, Shen X, et al. Legionella pneumophila inhibits macrophage apoptosis by targeting pro-death members of the Bcl2 protein family[J]. Proc Natl Acad Sci USA, 2007, 104(12): 5121-5126. DOI:10.1073/pnas.0611030104 |
| [19] |
惠英华, 杨志伟. 嗜肺军团菌致病性的研究进展[J]. 宁夏医科大学学报, 2012, 34(10): 1098-1100. DOI:10.3969/j.issn.1674-6309.2012.10.043 |
| [20] |
Rasch J, ünal CM, Klages A, et al. Peptidyl-prolyl-cis/trans-isomerases Mip and PpiB of Legionella pneumophila contri-bute to surface translocation, growth at suboptimal temperature, and infection[J]. Infect Immun, 2019, 87(1): e00939-17. |
| [21] |
Case CL, Kohler LJ, Lima JB, et al. Caspase -11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila[J]. Proc Natl Acad Sci USA, 2013, 110(5): 1851-1856. DOI:10.1073/pnas.1211521110 |
| [22] |
Palusińska-Szysz M, Russa R. Chemical structure and biological significance of lipopolysaccharide from Legionella[J]. Recent Pat Antiinfect Drug Discov, 2009, 4(2): 96-107. DOI:10.2174/157489109788490316 |
| [23] |
李凡, 徐志凯. 医学微生物学[M]. 9版. 北京: 人民卫生出版社, 2018.
|
| [24] |
Palusińska-Szysz M, Janczarek M. Innate immunity to Legionella and toll-like receptors -review[J]. Folia Microbiol, 2010, 55(5): 508-514. DOI:10.1007/s12223-010-0084-8 |
| [25] |
Albert-Weissenberger C, Cazalet C, Buchrieser C. Legionella pneumophila-a human pathogen that co-evolved with fresh water protozoa[J]. Cell Mol Life Sci, 2007, 64(4): 432-448. DOI:10.1007/s00018-006-6391-1 |
| [26] |
De Buck E, Anné J, Lammertyn E. The role of protein secretion systems in the virulence of the intracellular pathogen Legionella pneumophila[J]. Microbiol, 2007, 153(Pt 12): 3948-3953. |
| [27] |
Sola A, Lipoa E, de Jesús-Díaz DA, et al. Legionella pneumophila translocated translation inhibitors are required for bacterial-induced host cell cycle arrest[J]. Proc Natl Acad Sci USA, 2019, 116(8): 3221-3228. DOI:10.1073/pnas.1820093116 |
| [28] |
Schroeder GN, Aurass P, Oates CV, et al. Legionella pneumophila effector LpdA is a palmitoylated phospholipase D virulence factor[J]. Infect Immun, 2015, 83(10): 3989-4002. DOI:10.1128/IAI.00785-15 |
| [29] |
Zhan XY, Hu CH, Zhu QY. Legionella pathogenesis and viru-lence factors[J]. Ann Clin Lab Res, 2015, 3(2): 15. |
| [30] |
Cianciotto NP. Iron acquisition by Legionella pneumophila[J]. Biometals, 2007, 20(3-4): 323-331. DOI:10.1007/s10534-006-9057-4 |
| [31] |
Schulz T, Rydzewski K, Schunder E, et al. FliA expression analysis and influence of the regulatory proteins RpoN, FleQ and FliA on virulence and in vivo fitness in Legionella pneumophila[J]. Arch Microbiol, 2012, 194(12): 977-989. DOI:10.1007/s00203-012-0833-y |
| [32] |
Abu-Zant A, Asare R, Graham JE, et al. Role for RpoS but not RelA of Legionella pneumophila in modulation of phagosome biogenesis and adaptation to the phagosomal microenvironment[J]. Infect Immun, 2006, 74(5): 3021-3026. DOI:10.1128/IAI.74.5.3021-3026.2006 |
| [33] |
Fischer G, Bang H, Ludwig B, et al. Mip protein of Legionella pneumophila exhibits peptidyl-prolyl-cis/trans isomerase (PPIase) activity[J]. Mol Microbiol, 1992, 6(10): 1375-1383. DOI:10.1111/j.1365-2958.1992.tb00858.x |
| [34] |
Cirillo SL, Yan L, Littman M, et al. Role of the Legionella pneumophila rtxA gene in amoebae[J]. Microbiology, 2002, 148(Pt 6): 1667-1677. |
| [35] |
Liu M, Haenssler E, Uehara T, et al. The Legionella pneumophila EnhC protein interferes with immunostimulatory muramyl peptide production to evade innate immunity[J]. Cell Host Microbe, 2012, 12(2): 166-176. DOI:10.1016/j.chom.2012.06.004 |
| [36] |
Párraga-Niño N, Quero S, Uria N, et al. Antibody test for Legionella pneumophila detection[J]. Diagn Microbiol Infect Dis, 2018, 90(2): 85-89. DOI:10.1016/j.diagmicrobio.2017.11.005 |
| [37] |
Heidtman M, Chen EJ, Moy MY, et al. Large -scale identification of Legionella pneumophila Dot/Icm substrates that modulate host cell vesicle trafficking pathways[J]. Cell Microbiol, 2009, 11(2): 230-248. DOI:10.1111/j.1462-5822.2008.01249.x |
| [38] |
Swanson MS, Isberg RR. Identification of Legionella pneumophila mutants that have aberrant intracellular fates[J]. Infect Immun, 1996, 64(7): 2585-2594. |
| [39] |
Shohdy N, Efe JA, Emr SD, et al. Pathogen effector protein screening in yeast identifies Legionella factors that interfere with membrane trafficking[J]. Proc Natl Acad Sci USA, 2005, 102: 4866-4871. DOI:10.1073/pnas.0501315102 |
| [40] |
尹昆.嗜肺军团菌效应因子LidA对宿主Rabl的识别机制研究[D].济南: 山东大学, 2012.
|
| [41] |
肖婷, 赵桂华, 尹昆. 嗜肺军团菌调控宿主囊泡转运的效应因子及其分子机制[J]. 微生物学通报, 2016, 43(11): 2488-2494. |
| [42] |
Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion[J]. Cell, 2004, 116(2): 153-166. DOI:10.1016/S0092-8674(03)01079-1 |
| [43] |
Seabra MC, Wasmeier C. Controlling the location and activation of Rab GTPases[J]. Curr Opin Cell Biol, 2004, 16(4): 451-457. DOI:10.1016/j.ceb.2004.06.014 |
| [44] |
Hoffmann C, Finsel I, Otto A, et al. Functional analysis of novel Rab GTPases identified in the proteome of purified Legio- nella-containing vacuoles from macrophages[J]. Cell Micro -biol, 2014, 16(7): 1034-1052. |
| [45] |
Cheng W, Yin K, Lu D, et al. Structural insights into a unique Legionella pneumophila effector LidA recognizing both GDP and GTP bound Rab1 in their active state[J]. PLoS Pathog, 2012, 8(3): e1002528. DOI:10.1371/journal.ppat.1002528 |
| [46] |
Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle[J]. Dev Cell, 2007, 12(5): 671-682. DOI:10.1016/j.devcel.2007.04.005 |
| [47] |
Bugalhão JN, Mota LJ, Franco IS. Identification of regions within the Legionella pneumophila VipA effector protein involved in actin binding and polymerization and in interference with eukaryotic organelle trafficking[J]. Microbiologyopen, 2016, 5(1): 118-133. DOI:10.1002/mbo3.316 |
| [48] |
Ku B, Lee KH, Park WS, et al. VipD of Legionella pneumophila targets activated Rab5 and Rab22 to interfere with endosomal trafficking in macrophages[J]. PLoS Pathog, 2012, 8(12): e1003082. DOI:10.1371/journal.ppat.1003082 |
| [49] |
严慧, 朱庆义. 嗜肺军团菌主要毒力因子与细胞免疫反应[J]. 中华临床医师杂志(电子版), 2014, 8(2): 342-345. DOI:10.3877/cma.j.issn.1674-0785.2014.02.036 |
| [50] |
Haenssler E, Isberg RR. Control of host cell phosphorylation by Legionella pneumophila[J]. Front Microbiol, 2011, 2: 64. |
| [51] |
Chen J, de Felipe K, Clarke M, et al. Legionella effectors that promote nonlytic release from protozoa[J]. Science, 2004, 303(5662): 1358-1361. DOI:10.1126/science.1094226 |
| [52] |
Chen J, Reyes M, Clarke M, et al. Host cell-dependent secretion and translocation of the LepA and LepB effectors of Legio- nella[J]. Cell Microbiol, 2010, 9(7): 1660-1671. |
| [53] |
王嘉铭, 李向辉, 陈爱枫, 等. 嗜肺军团菌类真核效应蛋白LegK3抑制酿酒酵母的生长并影响其小泡运输途径[J]. 微生物学报, 2014, 54(4): 417-423. |
| [54] |
任君.嗜肺军团菌类真核效应蛋白的系统发生及生物学功能研究[D].广州: 中山大学, 2010. http://med.wanfangdata.com.cn/Paper/Detail/DegreePaper_Y1690429
|
| [55] |
Brüggemann H, Cazalet C, Buchrieser C. Adaptation of Legio- nella pneumophila to the host environment: role of protein secretion, effectors and eukaryotic-like proteins[J]. Curr Opin Microbiol, 2006, 9(1): 86-94. DOI:10.1016/j.mib.2005.12.009 |
| [56] |
Rolando M, Sanulli S, Rusniok C, et al. Legionella pneumophila effector RomA uniquely modifies host chromatin to repress gene expression and promote intracellular bacterial replication[J]. Cell Host Microbe, 2013, 13(4): 395-405. DOI:10.1016/j.chom.2013.03.004 |
| [57] |
Kubori T, Hyakutake A, Nagai H. Legionella translocates an E3 ubiquitin ligase that has multiple U-boxes with distinct functions[J]. Mol Microbiol, 2008, 67(6): 1307-1319. DOI:10.1111/j.1365-2958.2008.06124.x |
| [58] |
Brüggemann H, Hagman A, Jules M, et al. Virulence strategies for infecting phagocytes deduced from the in vivo transcriptional program of Legionella pneumophila[J]. Cell Microbiol, 2006, 8(8): 1228-1240. DOI:10.1111/j.1462-5822.2006.00703.x |
| [59] |
Ren T, Zamboni DS, Roy CR, et al. Flagellin-deficient Legionella mutants evade caspase -1 and Naip5-mediated macrophage immunity[J]. PloS Pathog, 2006, 2(3): e18. DOI:10.1371/journal.ppat.0020018 |
| [60] |
Silveira TN, Zamboni DS. Pore formation triggered by Legionella spp. is an Nlrc4 inflammasome -dependent host cell response that precedes pyroptosis[J]. Infect Immun, 2010, 78(3): 1403-1413. DOI:10.1128/IAI.00905-09 |
| [61] |
Xu L, Shen X, Bryan A, et al. Inhibition of host vacuolar H+-ATPase activity by a Legionella pneumophila effector[J]. PloS Pathog, 2010, 6(3): e1000822. DOI:10.1371/journal.ppat.1000822 |
| [62] |
Yao J, Yang F, Sun X, et al. Mechanism of inhibition of retro - mer transport by the bacterial effector RidL[J]. Proc Natl Acad Sci USA, 2018, 115(7): e1446-e1454. DOI:10.1073/pnas.1717383115 |
| [63] |
Bhogaraju S, Kalayil S, Liu Y, et al. Phosphoribosylation of ubiquitin promotes serine ubiquitination and impairs conventional ubiquitination[J]. Cell, 2016, 167(6): 1636-1649. DOI:10.1016/j.cell.2016.11.019 |
| [64] |
Mou Q. Interactions of Legionella pneumophila with amoeba and human hosts: cellular and molecular mechanisms[D]. Hong Kong: the Hong Kong Polytechnic University, 2019.
|
| [65] |
Amor JC, Swails J, Zhu X, et al. The structure of RalF, an ADP-ribosylation factor guanine nucleotide exchange factor from Legionella pneumophila, reveals the presence of a cap over the active site[J]. J Biol Chem, 2005, 280(2): 1392-1400. DOI:10.1074/jbc.M410820200 |
| [66] |
Creasey EA, Isberg RR. The protein SdhA maintains the integrity of the Legionella-containing vacuole[J]. Proc Natl Acad Sci USA, 2012, 109(9): 3481-3486. DOI:10.1073/pnas.1121286109 |
| [67] |
Hsu FS, Zhu W, Brennan L, et al. Structural basis for substrate recognition by a unique Legionella phosphoinositide phosphatase[J]. Proc Natl Acad Sci USA, 2012, 109(34): 13567-13572. DOI:10.1073/pnas.1207903109 |
| [68] |
Shen X, Banga S, Liu Y, et al. Targeting eEF1A by a Legionella pneumophila effector leads to inhibition of protein synthesis and induction of host stress response[J]. Cell Micro-biol, 2009, 11(6): 911-926. DOI:10.1111/j.1462-5822.2009.01301.x |
| [69] |
Belyi Y, Tabakova I, Stahl M, et al. Lgt: a family of cytotoxic glucosyltransferases produced by Legionella pneumophila[J]. J Bacteriol, 2008, 190(8): 3026-3035. DOI:10.1128/JB.01798-07 |
| [70] |
Isaac DT, Laguna RK, Valtz N, et al. MavN is a Legionella pneumophila vacuole -associated protein required for efficient iron acquisition during intracellular growth[J]. Proc Natl Acad Sci USA, 2015, 112(37): e5208-e5217. DOI:10.1073/pnas.1511389112 |
| [71] |
Portier E, Zheng H, Sahr T, et al. IroT/mavN, a new iron-regulated gene involved in Legionella pneumophila virulence against amoebae and macrophages[J]. Environ Microbiol, 2015, 17(4): 1338-1350. DOI:10.1111/1462-2920.12604 |
| [72] |
Allgood SC, Romero Dueñas BP, Noll RR, et al. Legionella effector AnkX disrupts host cell endocytic recycling in a phosphocholination-dependent manner[J]. Front Cell Infect Microbiol, 2017, 7: 397. DOI:10.3389/fcimb.2017.00397 |
| [73] |
Choy A, Dancourt J, Mugo B, et al. The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation[J]. Science, 2012, 338(6110): 1072-1076. DOI:10.1126/science.1227026 |



