2. 四川大学护理学四川省重点实验室,四川 成都 610041
2. Sichuan Key Laboratory of Nursing, Sichuan University, Chengdu 610041, China
手术部位感染(surgical site infection, SSI)是一种与任何类型外科手术相关的潜在并发症[1-2]。据世界卫生组织(WHO)调查,SSI是低收入和中等收入国家最常见的医院感染类型,影响多达1/3的手术患者[3]。加速康复外科理念是由欧洲外科医生小组2001年提出,其主要目的是减少患者术后住院时间,促进术后迅速恢复正常活动,从而降低并发症发生率和手术相关费用[4]。关节置换术是最常见的外科手术,占所有手术的33%[5]。据报道[6-9],全膝关节置换术(total knee arthroplasty, TKA)的术后感染率为1%~3%,全髋关节置换术(total hip arthroplasty, THA)的术后感染率为0.7%~2.5%。SSI可能导致髋、膝关节置换术患者住院时间延长以及二次手术,给患者带来沉重的经济负担,阻碍康复进程[10-11],因此,预防髋、膝关节置换术后SSI对于加速患者的康复十分必要。目前国内外已有预防SSI的相关指南,但存在涉及的手术广泛,未聚焦至髋、膝关节置换术,且不同国家在解释证据和推荐意见方面不一致等问题。本研究通过系统检索、科学评价及总结髋、膝关节置换术后预防SSI的相关证据,旨在根据我国国情为髋、膝关节置换术术前、术中和术后预防SSI提出循证建议。
1 方法 1.1 提出问题根据PIPOST原则构建循证护理问题:P(population)为证据应用的目标人群,指髋、膝关节置换的患者;I(intervention)为干预措施,包括手术前、手术中、手术后的预防措施;P(professional)为应用证据的专业人员,指医务人员;O(outcome)为结局指标,即手术部位感染率;S(setting)为证据应用场所,医院骨科病房、手术室等;T(type of evidence)为证据类型,包括循证指南、临床实践、专家共识、系统评价等。
1.2 检索策略英文检索词包括“surgery site infection OR periprosthetic joint infection”AND“total knee arthroplasty OR total knee replacement OR TKA OR total hip arthroplasty OR total hip replacement OR THA OR total joint arthroplasty OR total joint replacement OR TJA”AND“best practice OR guideline OR evidence OR practical guidance OR consensus OR systematic review OR Meta-analysis”,中文检索词为“手术部位感染/假体周围感染”和“全膝关节置换术/全髋关节置换术/关节置换术”,限制文献类型为指南、专家共识、临床实践或系统评价。检索计算机决策支持系统BMJ Best Practice、Up To Date,检索指南网包括BMJ Clinical Evidence、WHO、国际指南协作网(GIN)、英国国家卫生与临床优化研究所(NICE)、美国国立指南库(NGC)、加拿大安大略医学会(RNAO)、苏格兰院际间指南网(SIGN)、新西兰指南协作组(NZGG)、美国医师学会俱乐部ACP Club。检索数据库包括Cochrane临床对照试验中心注册数据库、JBI循证卫生保健知识库、PubMed、Embase、CINAHL、中国知网、万方数据库、中国生物医学文献数据库以及欧洲灰色文献信息系统SIGLE等。检索日期为2016年12月—2021年12月。
1.3 文献纳入及排除标准纳入标准:研究对象为年龄>18岁的髋、膝关节置换术患者;结局指标为髋、膝关节置换术后发生SSI;研究类型为循证指南、专家共识、临床实践、系统评价等;没有语言限制,所有文献均是5年以内发表的文献。排除标准:指南解读、综述、病例对照研究、病例报告、致编辑信函、病例系列;无法获取全文的文献。
1.4 证据质量的评估 1.4.1 质量评估工具(1) 使用2012版临床指南研究与评价系统(Appraisal of Guidelines for Research and Evaluation Instrument, Agree Ⅱ)进行量化评分,该工具有27个条目,包括6个领域和2个总体评估项[12]。每个条目为1~7分,1分代表该指南完全不符合该条目,7分代表完全符合,分数越高,指南质量越好。强烈推荐(A级)要求指南6个领域得分均≥60%;中度推荐(B级)要求3个及以上的领域得分≥30%,但有<60%的领域;3个及以上的领域得分<30%为不推荐(C级)。(2)使用澳大利亚JBI循证卫生保健中心(2016)对专家意见和专业共识类文献的质量评价工具[13]评价专家共识,包含6个评价项目。每个评价项目的判断结果包括“是”、“否”、“不清楚”、“不适用”。(3)采用系统综述评价工具(assessment of multiple systematic reviews, AMSTAR)[13]对系统评价和Meta分析进行评价。该工具共11个条目,分为“是”、“否”、“不清楚”和“未提及”4个评价选项。
1.4.2 质量评价过程由2名学习过循证医学系列课程的研究生进行证据的质量评价,评价者之间的争议通过第3位研究者讨论解决,当不同文献的证据结论相互冲突时,研究者依从循证证据优先、高质量证据优先、最新发布的权威文献优先原则进行推荐。
根据2014版JBI证据预分级和证据推荐级别系统[14],首先对研究设计进行预分级,分为Level 1~5五个等级,Level 1为最高级别;其次在JBI FAME结构的指导下,考虑证据的有效性、可行性、适宜性、临床意义4个方面,再结合JBI证据推荐级别系统,对证据进行推荐(A级推荐为强推荐、B级推荐为弱推荐)。
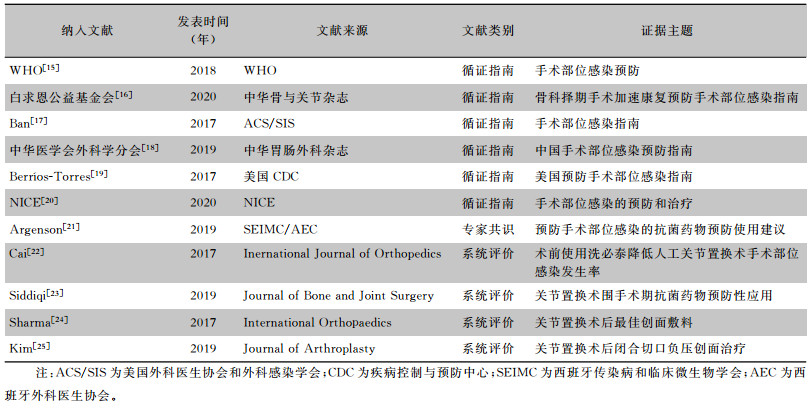
2 结果 2.1 证据检索结果本研究共检索到389篇文献,在审查标题和摘要后,剔除重复和不符合主题的研究127篇,2名研究者再次对40篇文献进行全文筛查,排除不符合纳入标准的文献(21篇)和文献质量评价不过关的文献(8篇)后,最终确定纳入11项研究,其中包括6篇循证指南、1篇专家共识、4篇系统评价。纳入文献的一般特征见表 1,文献筛选流程见图 1。
| 表 1 加速康复理念下髋、膝关节置换术SSI预防的最佳证据总结纳入文献的一般特征 Table 1 General characteristics of included literatures on the best evidence for the prevention of SSI after hip and knee arthroplasty under the concept of enhanced recovery |
|
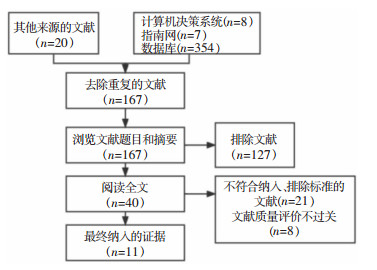 |
| 图 1 加速康复理念下髋膝关节置换术后手术部位感染预防的最佳证据总结筛选流程 Figure 1 Flow chart of evidences screening for the best evidence for the prevention of SSI after hip and knee arthroplasty under the concept of enhanced reco-very |
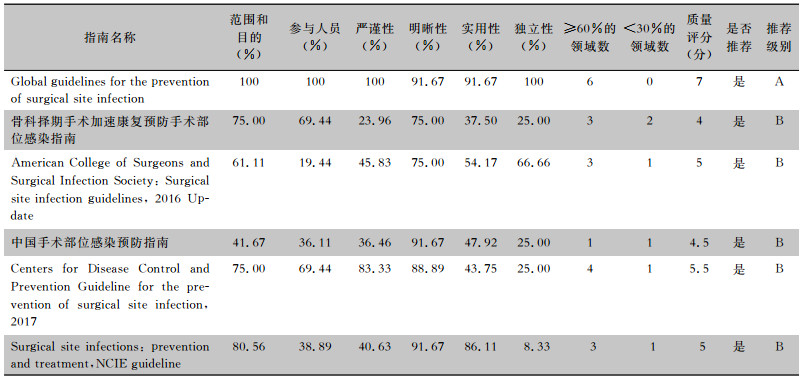
本研究共纳入6篇[15-20]循证指南,分别来自WHO、中国骨与关节杂志、ACS/SIS、中国胃肠外科杂志、美国CDC、NICE。其质量评价结果见表 2。
| 表 2 6篇循证指南的Agree Ⅱ评分结果 Table 2 Agree Ⅱ score of 6 evidence-based guidelines |
|
本研究纳入1篇SEIMC/AEC发表的专家共识[21],4篇来自于“PubMed”的系统评价[22-25]。其中,专家共识所有条目的评价结果均为“是”。研究设计完整,整体质量高,准予纳入。2篇系统评价[22, 25]的所有条目评价结果均为“是”。研究设计完整,整体质量高,准予纳入。Sharma等[24]研究中除条目7(是否评价和报道纳入研究的科学性)和条目10(是否评估发表偏倚)为“否”外,其他条目的评价结果均为“是”,研究设计较为完整,整体质量较高,准予纳入。Siddiqi等[23]的研究中除条目11(是否说明相关利益冲突)为“否”外,其他条目评价结果均为“是”,研究设计比较完整,整体质量较高,准予纳入。
2.3 最佳证据总结通过总结提取所有入选的证据,加速康复外科理念下髋、膝关节置换术后SSI的证据总结包括术前、术中和术后3个方面,共23条推荐意见,其中A级推荐11条,B级推荐12条。髋、膝关节置换术SSI预防的最佳证据总结见表 3。
| 表 3 髋、膝关节置换术SSI预防的最佳证据总结 Table 3 Summary of the best evidence for prevention of SSI in hip and knee arthroplasty |

|
共有10条髋、膝关节置换术后SSI的术前预防证据。其中第2条证据“术前对潜在感染灶筛查”,感染灶筛查包括详细询问、体格检查和实验室筛查,证据来源较少,无高质量的研究证明术前进行实验室筛查可以预防SSI。相反,有研究[26]指出,术前常规筛查产超广谱β-内酰胺酶(extended-spectrum beta-lactamase, ESBLs)肠杆菌可能会增加ESBLs定植患者术前广泛使用广谱抗菌药物(尤其是碳青霉烯类),从而进一步增加革兰阴性菌,尤其是耐碳青霉烯类肠杆菌的耐药性,需要高质量的研究进一步比较术前细菌筛查与术前不进行筛查对预防SSI的影响。第4条证据“在手术切皮前120 min内预防性使用抗菌药物,不建议使用含抗菌药物的骨水泥减少SSI”,此外使用抗菌药物的时间应考虑药物半衰期,确定给药时间,使用止血带的手术应在止血带充气前10 min内使用完抗菌药物。第7条证据“不建议为预防SSI在手术切口使用任何抗菌密封剂或抗菌药物”,手术部位皮肤准备后不应使用抗菌密封剂,不要在手术切口上涂抹抗菌药物(如软膏、溶液或粉剂等)以防止SSI。较低质量的研究[27]表明除标准的手术部位皮肤准备外,术前应用抗菌皮肤密封剂已被证明可以减少皮肤上的细菌数量,但是在降低SSI发病率方面既未产生积极作用,也无消极作用[28-31]。WHO专家小组建议不应在手术部位使用皮肤抗菌密封剂,因为皮肤刺激和过敏反应可能与使用抗菌密封剂有关[32]。第9条证据“加强营养,纠正贫血”,主要是预防体重过轻患者在接受大手术后发生SSI。其中体重过轻的患者定义为身体质量指数低于18.5或体重低于其年龄和身高标准体重的15%~20%。研究[33-34]认为,营养干预可以降低SSI发病率,因为免疫系统可以通过营养支持来调节。有研究[35]表明,骨科植入手术前清蛋白水平过低的患者,会导致术后感染风险增加,较低质量研究[36]建议,术前清蛋白水平纠正到35 g/L以上可以预防感染,建议加强髋、膝关节置换术患者术前的营养,提高患者抵抗力[37]。其中,第10条证据“不建议以预防SSI为目的停用免疫抑制剂”。一项观察性研究[38]表明,免疫抑制药物可能会导致使用这些药物治疗的患者切口愈合受损,感染风险增加。但也有研究[39-40]表明,使用免疫抑制剂治疗类风湿性关节炎的患者中,与停药相关不良事件的风险很高。非常低质量的证据表明,与继续使用免疫抑制剂相比,围手术期停用甲氨蝶呤对类风湿性关节炎患者SSI的影响尚无定论[41]。因此还需要进一步研究停用免疫抑制剂的最佳时间,此外,还应研究各种免疫抑制剂的最佳剂量对SSI发病率的影响。
共有10条髋、膝关节置换术后SSI的术中预防证据。第16条证据“不建议在手术部位贴有无抗菌性的塑料手术薄膜”,理论上认为,关节置换手术部位的塑料贴膜形成一种机械和/或微生物屏障,以防止微生物从皮肤迁移到手术部位[42]。然而,系统评价[43-44]表明使用塑料薄膜并不能降低SSI发病率,使用含碘伏的塑料贴膜可能还与患者出现过敏有关,胶膜碎片和黏合剂很可能会残留在切口[45],因为涉及的研究质量非常低,还需要更多设计良好的随机对照试验进一步调查这些产品的潜在好处。第17条证据“建议考虑在切口闭合前用聚维酮碘水溶液冲洗切口”,以防止SSI。特别是受污染的切口,不建议在切口闭合前使用抗菌药物冲洗以预防SSI[15]。低质量研究[46]表明,与生理盐水冲洗相比,用聚维酮碘溶液冲洗切口有利于降低SSI风险,目前还无证据表明聚维酮碘溶液的最佳使用浓度。研究[47]表明,与不使用抗菌药物或生理盐水冲洗相比,切口抗菌药物冲洗在减少SSI方面既不产生积极作用,也不产生消极作用。第20条建议“不建议使用层流通风系统”,因为来自层流系统的新鲜空气对手术切口和患者的冷却作用可能导致手术切口组织温度降低,如果术中不监测患者体温,则可能导致全身体温过低[48],2012年发表的一篇关于层流气流对假体关节感染影响的系统综述发现,层流气流通气是发生严重SSI的危险因素[49]。一项观察性研究[50]评估了手术室自然通气与层流通气对髋、膝关节置换术后SSI的影响发现,两者SSI发病率差异无统计学意义。
共有3条髋、膝关节置换术后SSI的术中预防证据。其中第22条证据“切口处使用高级敷料或者标准敷料时,SSI发病率相同”。研究表明,与标准敷料相比,银离子敷料并不能显著降低SSI发病率[51],但可以降低切口起泡率、渗漏率,减少敷料更换次数。低质量证据[52]表明,水胶体敷料比标准敷料更舒适。未来还需要大样本量、高质量的研究探讨含银敷料对髋、膝关节置换术后手术切口的影响,此外,还应探讨不透明敷料与透明敷料在术后视觉检查和敷料使用时间在预防SSI方面的作用。
髋、膝关节置换术后发生SSI,延长患者住院时间、增加医疗负担,阻碍加速康复外科的开展。本研究总结目前国内外现存的髋、膝关节置换术后预防SSI的最佳证据,证据主要来源于各国专业的指南网和专家共识,证据质量高,可信度和临床应用价值较高,可以为骨外科团队,即骨科医生、护士、麻醉师等提供临床参考,为应用预防SSI的术前、术中和术后的干预措施提供全面的循证建议。但是由于涉及的指南和系统评价来源于欧美国家的偏多,建议应用证据之前根据我国的文化、资源、医院的条件和患者本身(年龄、身体状况等)精准应用证据,优化围手术期预防髋、膝关节置换术后SSI的措施,以利于保障患者安全和提高医疗服务质量。
利益冲突:所有作者均声明不存在利益冲突。
| [1] |
Haley RW, Culver DH, White JW, et al. The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals[J]. Am J Epidemiol, 1985, 121(2): 182-205. DOI:10.1093/oxfordjournals.aje.a113990 |
| [2] |
Harbarth S, Sax H, Gastmeier P. The preventable proportion of nosocomial infections: an overview of published reports[J]. J Hosp Infect, 2003, 54(4): 258-266. DOI:10.1016/S0195-6701(03)00150-6 |
| [3] |
Allegranzi B, Bagheri Nejad S, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and Meta-analysis[J]. Lancet, 2011, 377(9761): 228-241. DOI:10.1016/S0140-6736(10)61458-4 |
| [4] |
Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome[J]. Am J Surg, 2002, 183(6): 630-641. DOI:10.1016/S0002-9610(02)00866-8 |
| [5] |
World Health Organization. WHO guidelines for safe surgery 2009: safe surgery saves lives[EB/OL]. [2022-03-19]. https://apps.who.int/iris/handle/10665/44185.
|
| [6] |
Bozic KJ, Grosso LM, Lin ZQ, et al. Variation in hospital-level risk-standardized complication rates following elective primary total hip and knee arthroplasty[J]. J Bone Joint Surg Am, 2014, 96(8): 640-647. DOI:10.2106/JBJS.L.01639 |
| [7] |
Frank RM, Cross MB, Della Valle CJ. Periprosthetic joint infection: modern aspects of prevention, diagnosis, and treatment[J]. J Knee Surg, 2015, 28(2): 105-112. |
| [8] |
Maoz G, Phillips M, Bosco J, et al. The Otto Aufranc Award: modifiable versus nonmodifiable risk factors for infection after hip arthroplasty[J]. Clin Orthop Relat Res, 2015, 473(2): 453-459. DOI:10.1007/s11999-014-3780-x |
| [9] |
Tsung JD, Rohrsheim JAL, Whitehouse SL, et al. Management of periprosthetic joint infection after total hip arthroplasty using a custom made articulating spacer (CUMARS); the Exeter experience[J]. J Arthroplasty, 2014, 29(9): 1813-1818. DOI:10.1016/j.arth.2014.04.013 |
| [10] |
National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004[J]. Am J Infect Control, 2004, 32(8): 470-485. DOI:10.1016/j.ajic.2004.10.001 |
| [11] |
Peel TN, Buising KL, Choong PFM. Prosthetic joint infection: challenges of diagnosis and treatment[J]. ANZ J Surg, 2011, 81(1-2): 32-39. DOI:10.1111/j.1445-2197.2010.05541.x |
| [12] |
Brouwers MC, Kho ME, Browman GP, et al. AGREE Ⅱ: advancing guideline development, reporting and evaluation in health care[J]. CMAJ, 2010, 182(18): E839-E842. DOI:10.1503/cmaj.090449 |
| [13] |
熊俊, 陈日新. 系统评价/Meta分析方法学质量的评价工具AMSTAR[J]. 中国循证医学杂志, 2011, 11(9): 1084-1089. Xiong J, Chen RX. An introduction to a measurement tool to assess the methodological quality of systematic reviews/Meta-analysis: AMSTAR[J]. Chinese Journal of Evidence-Based Medicine, 2011, 11(9): 1084-1089. DOI:10.3969/j.issn.1672-2531.2011.09.017 |
| [14] |
王春青, 胡雁. JBI证据预分级及证据推荐级别系统(2014版)[J]. 护士进修杂志, 2015, 30(11): 964-967. Wang CQ, Hu Y. JBI evidence pre-classification and evidence rank system(2014 edition)[J]. Journal of Nurses Training, 2015, 30(11): 964-967. |
| [15] |
World Health Organization. Global guidelines for the prevention of surgical site infection, 2nd ed[EB/OL]. [2022-03-19]. https://apps.who.int/iris/handle/10665/277399.
|
| [16] |
白求恩公益基金会, 中国康复技术转化及发展促进会, 中国研究型医院学会, 等. 骨科择期手术加速康复预防手术部位感染指南[J]. 中华骨与关节外科杂志, 2020, 13(1): 1-7. Bethune Charitable Foundation, China Association of Rehabilitation Technology Transformation and Promotion, Chinese Research Hospital Association, et al. Guideline on the prevention of surgical site infection for the enhanced recovery after surgery after elective orthopaedic surgeries[J]. Chinese Journal of Bone and Joint Surgery, 2020, 13(1): 1-7. DOI:10.3969/j.issn.2095-9958.2020.01.01 |
| [17] |
Ban KA, Minei JP, Laronga C, et al. American College of Surgeons and Surgical Infection Society: surgical site infection guidelines, 2016 update[J]. J Am Coll Surg, 2017, 224(1): 59-74. DOI:10.1016/j.jamcollsurg.2016.10.029 |
| [18] |
中华医学会外科学分会外科感染与重症医学学组, 中国医师协会外科医师分会肠瘘外科医师专业委员会. 中国手术部位感染预防指南[J]. 中华胃肠外科杂志, 2019, 22(4): 301-314. Chinese Society of Surgical Infection and Intensive Care, Chinese Society of Surgery, Chinese Medical Association, Chinese College of Gastrointestinal Fistula Surgeons, Chinese College of Surgeons, Chinese Medical Doctor Association. Chinese guideline for the prevention of surgical site infection[J]. Chinese Journal of Gastrointestinal Surgery, 2019, 22(4): 301-314. |
| [19] |
Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017[J]. JAMA Surg, 2017, 152(8): 784-791. DOI:10.1001/jamasurg.2017.0904 |
| [20] |
National Institute for Health and Care Excellence. Surgical site infections: prevention and treatment[EB/OL]. (2020-08-19)[2022-03-19]. https://www.nice.org.uk/guidance/ng125.
|
| [21] |
Argenson JN, Arndt M, Babis G, et al. Hip and knee section, treatment, debridement and retention of implant: proceedings of international consensus on orthopedic infections[J]. J Arthroplasty, 2019, 34(2S): S399-S419. |
| [22] |
Cai YZ, Xu K, Hou WK, et al. Preoperative chlorhexidine reduces the incidence of surgical site infections in total knee and hip arthroplasty: a systematic review and Meta-analysis[J]. Int J Surg, 2017, 39: 221-228. DOI:10.1016/j.ijsu.2017.02.004 |
| [23] |
Siddiqi A, Forte SA, Docter S, et al. Perioperative antibiotic prophylaxis in total joint arthroplasty: a systematic review and Meta-analysis[J]. J Bone Joint Surg Am, 2019, 101(9): 828-842. DOI:10.2106/JBJS.18.00990 |
| [24] |
Sharma G, Lee SW, Atanacio O, et al. In search of the optimal wound dressing material following total hip and knee arthroplasty: a systematic review and Meta-analysis[J]. Int Orthop, 2017, 41(7): 1295-1305. DOI:10.1007/s00264-017-3484-4 |
| [25] |
Kim JH, Kim HJ, Lee DH. Comparison of the efficacy between closed incisional negative-pressure wound therapy and conventional wound management after total hip and knee arthroplasties: a systematic review and Meta-analysis[J]. J Arthroplasty, 2019, 34(11): 2804-2814. DOI:10.1016/j.arth.2019.06.020 |
| [26] |
Tacconelli E, Sifakis F, Harbarth S, et al. Surveillance for control of antimicrobial resistance[J]. Lancet Infect Dis, 2018, 18(3): e99-e106. DOI:10.1016/S1473-3099(17)30485-1 |
| [27] |
Dohmen PM. Impact of antimicrobial skin sealants on surgical site infections[J]. Surg Infect (Larchmt), 2014, 15(4): 368-371. DOI:10.1089/sur.2012.193 |
| [28] |
Daeschlein G, Napp M, Assadian O, et al. Influence of preoperative skin sealing with cyanoacrylate on microbial contamination of surgical wounds following trauma surgery: a prospective, blinded, controlled observational study[J]. Int J Infect Dis, 2014, 29: 274-278. DOI:10.1016/j.ijid.2014.08.008 |
| [29] |
Doorly M, Choi J, Floyd A, et al. Microbial sealants do not decrease surgical site infection for clean-contaminated colorectal procedures[J]. Tech Coloproctol, 2015, 19(5): 281-285. DOI:10.1007/s10151-015-1286-5 |
| [30] |
Dromzee E, Tribot-Laspière Q, Bachy M, et al. Efficacy of integuseal for surgical skin preparation in children and adolescents undergoing scoliosis correction[J]. Spine (Phila Pa 1976), 2012, 37(21): E1331-E1335. DOI:10.1097/BRS.0b013e3182687d6c |
| [31] |
Iyer A, Gilfillan I, Thakur S, et al. Reduction of surgical site infection using a microbial sealant: a randomized trial[J]. J Thorac Cardiovasc Surg, 2011, 142(2): 438-442. DOI:10.1016/j.jtcvs.2011.02.014 |
| [32] |
Towfigh S, Cheadle WG, Lowry SF, et al. Significant reduction in incidence of wound contamination by skin flora through use of microbial sealant[J]. Arch Surg, 2008, 143(9): 885-891. DOI:10.1001/archsurg.143.9.885 |
| [33] |
Culebras JM. Malnutrition in the twenty-first century: an epidemic affecting surgical outcome[J]. Surg Infect (Larchmt), 2013, 14(3): 237-243. DOI:10.1089/sur.2013.9993 |
| [34] |
Culebras-Fernández JM, de Paz-Arias R, Jorquera-Plaza F, et al. Nutrition in the surgical patient: immunonutrition[J]. Nutr Hosp, 2001, 16(3): 67-77. |
| [35] |
Rasouli MR, Restrepo C, Maltenfort MG, et al. Risk factors for surgical site infection following total joint arthroplasty[J]. J Bone Joint Surg Am, 2014, 96(18): e158. DOI:10.2106/JBJS.M.01363 |
| [36] |
Namba RS, Inacio MCS, Paxton EW. Risk factors associated with surgical site infection in 30, 491 primary total hip replacements[J]. J Bone Joint Surg Br, 2012, 94(10): 1330-1338. |
| [37] |
周宗科, 翁习生, 曲铁兵, 等. 中国髋、膝关节置换术加速康复——围术期管理策略专家共识[J]. 中华骨与关节外科杂志, 2016, 9(1): 1-9. Zhou ZK, Weng XS, Qu TB, et al. Expert consensus in enhanced recovery after total hip and knee arthroplasty in China: perioperative management[J]. Chinese Journal of Bone and Joint Surgery, 2016, 9(1): 1-9. DOI:10.3969/j.issn.2095-9985.2016.01.001 |
| [38] |
Berthold E, Geborek P, Gülfe A. Continuation of TNF blockade in patients with inflammatory rheumatic disease. An observational study on surgical site infections in 1, 596 elective orthopedic and hand surgery procedures[J]. Acta Orthop, 2013, 84(5): 495-501. DOI:10.3109/17453674.2013.842431 |
| [39] |
Murata K, Yasuda T, Ito H, et al. Lack of increase in postoperative complications with low-dose methotrexate therapy in patients with rheumatoid arthritis undergoing elective orthopedic surgery[J]. Mod Rheumatol, 2006, 16(1): 14-19. DOI:10.3109/s10165-005-0444-4 |
| [40] |
Sany J, Anaya JM, Canovas F, et al. Influence of methotrexa-te on the frequency of postoperative infectious complications in patients with rheumatoid arthritis[J]. J Rheumatol, 1993, 20(7): 1129-1132. |
| [41] |
den Broeder AA, Creemers MCW, Fransen J, et al. Risk factors for surgical site infections and other complications in elective surgery in patients with rheumatoid arthritis with special attention for anti-tumor necrosis factor: a large retrospective study[J]. J Rheumatol, 2007, 34(4): 689-695. |
| [42] |
French ML, Eitzen HE, Ritter MA. The plastic surgical adhesive drape: an evaluation of its efficacy as a microbial barrier[J]. Ann Surg, 1976, 184(1): 46-50. DOI:10.1097/00000658-197607000-00008 |
| [43] |
Webster J, Alghamdi A. Use of plastic adhesive drapes during surgery for preventing surgical site infection[J]. Cochrane Database Syst Rev, 2013(1): CD006353. |
| [44] |
Webster J, Alghamdi A. Use of plastic adhesive drapes during surgery for preventing surgical site infection[J]. Cochrane Database Syst Rev, 2015, 2015(4): CD006353. |
| [45] |
Zokaie S, White IR, McFadden JD. Allergic contact dermatitis caused by iodophor-impregnated surgical incise drape[J]. Contact Dermatitis, 2011, 65(5): 309. DOI:10.1111/j.1600-0536.2011.01965.x |
| [46] |
Cheng MT, Chang MC, Wang ST, et al. Efficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgery[J]. Spine (Phila Pa 1976), 2005, 30(15): 1689-1693. DOI:10.1097/01.brs.0000171907.60775.85 |
| [47] |
Tanaka K, Matsuo K, Kawaguchi D, et al. Randomized clinical trial of peritoneal lavage for preventing surgical site infection in elective liver surgery[J]. J Hepatobiliary Pancreat Sci, 2015, 22(6): 446-453. DOI:10.1002/jhbp.222 |
| [48] |
Yang L, Huang CY, Zhou ZB, et al. Risk factors for hypothermia in patients under general anesthesia: Is there a drawback of laminar airflow operating rooms? A prospective cohort study[J]. Int J Surg, 2015, 21: 14-17. DOI:10.1016/j.ijsu.2015.06.079 |
| [49] |
Gastmeier P, Breier AC, Brandt C. Influence of laminar airflow on prosthetic joint infections: a systematic review[J]. J Hosp Infect, 2012, 81(2): 73-78. DOI:10.1016/j.jhin.2012.04.008 |
| [50] |
Song KH, Kim ES, Kim YK, et al. Differences in the risk factors for surgical site infection between total hip arthroplasty and total knee arthroplasty in the Korean Nosocomial Infections Surveillance System (KONIS)[J]. Infect Control Hosp Epidemiol, 2012, 33(11): 1086-1093. DOI:10.1086/668020 |
| [51] |
Burke NG, Green C, McHugh G, et al. A prospective randomised study comparing the jubilee dressing method to a standard adhesive dressing for total hip and knee replacements[J]. J Tissue Viability, 2012, 21(3): 84-87. DOI:10.1016/j.jtv.2012.04.002 |
| [52] |
Wynne R, Botti M, Stedman H, et al. Effect of three wound dressings on infection, healing comfort, and cost in patients with sternotomy wounds: a randomized trial[J]. Chest, 2004, 125(1): 43-49. DOI:10.1378/chest.125.1.43 |



